Describe How Red Blood Cells Use Osmosis
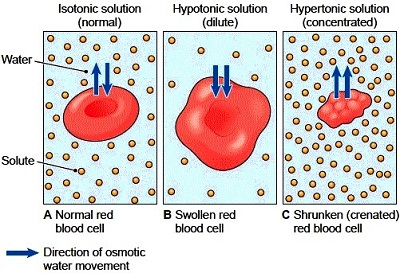
Osmosis in Red Blood Cells In the diagram below Red Blood Cells have been subjected to different conditions that exist in the human body. A red blood cell has what is known as a biconcave shape.
What Are Diffusion And Osmosis Enotes Com
If the soil is wet or moist root hair cells will also take up water by osmosis.

. For example red blood cells. Because of osmosis the water in the blood and very small molecules of waste move across the membrane into the dialysis fluid. Eventually the dialysis fluid will remove all of the waste materials it.
It is important for a cells survival to regulate osmosis in order to maintain an optimal internal environment according to Student Study Guide for Campbells BIOLOGY Fourth Edition by Martha R. Ovis Aries red blood cells were immersed. Three termshyerptonic hypotonic and isotonicare used to describe whether a solution will cause water to move into or out of a cell.
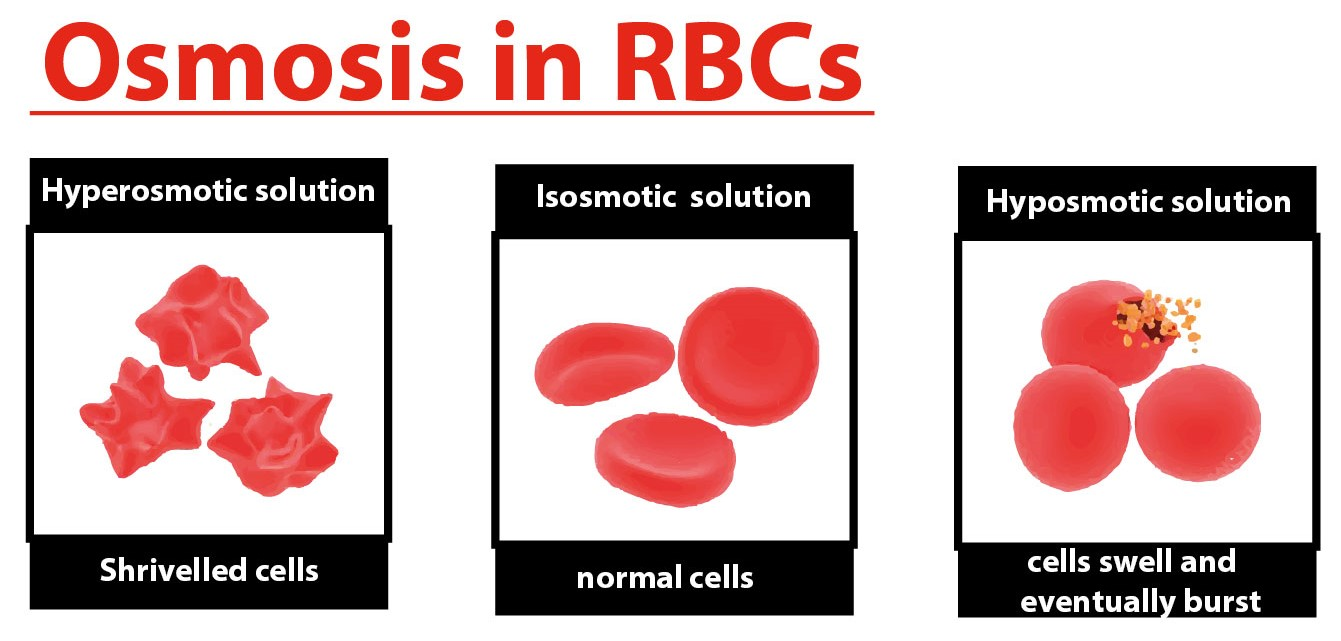
Examine the mount under low medium and high power. Observe the red blood cells until no further change occurs. This results in crenation shriveling of the blood cellWater moves into and out of cells by osmosis.
The roots of the plant have a higher solute concentration than the surrounding soil so water flows into the roots. By placing red blood cells in solutions of differing osmolarities and tonicities this experiment demonstrates the effects of osmosis and the resultant changes in cell volume. Red blood cells behave the same way see figure below.
Net water movement continues until its potential reaches zero. As far as red blood cells are concerned osmosis is a critical process because it ultimately determines healthiness. Listed below are more examples of Osmosis.
Chemistry Daily 2007 A 09 NaCl an isotonic solution and when Red Blood Cells reside in such a medium the intracellular and extracellular fluids are in osmotic equilibrium across the cell membrane causing no net influx or efflux of water. Describe the appearance of individual red blood cells. Osmosis is how plants are able to absorb water from soil.
The red blood cells therefore lose their normal biconcave shape and shrink or crenate. Using hemoglobin standard solutions where known concentrations of hemoglobin are produced the proportion of hemolysis and the effect of this on resultant hematocrit can be estimated. This movement occurs through osmosis because the cell has more free water than the solution.
In an animal cell osmosis helps in absorbing water from the intestines to the blood. In the case of mammalian red blood cells the plasma membrane is incredibly permeable to water and osmosis across the cell membrane occurs swiftly with sensitivity to osmotic pressure Freeman 2000. Osmotic pressure is the force required to prevent water movement across the semipermeable membrane.
Large quantities of water molecules constantly move across cell membranes by simple diffusion often facilitated by movement through membrane proteins including aquaporins. Osmosis and cells play integral roles in biological life. Red blood cells also called erythrocytes are the most abundant cell type in the blood.
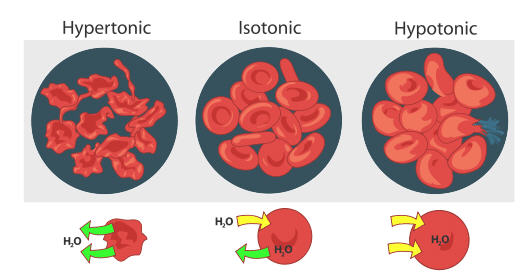
Osmosis is the traveling of water across a membrane. A solution will be hypertonic to a cell if its solute concentration is higher. If a cell is placed in a hypertonic solution there will be a net flow of water out of the cell and the cell will lose volume.
Describe what you think happened in the body to result in the three conditions. For example it has been estimated that an amount of water equivalent to roughly. Being that blood cells are selectively permeably decides what is allowednot allowed inside the ways in which water travel in and out of the cells is important.
When red blood cells are in a hypertonic higher concentration solution water flows out of the cell faster than it comes in. Hemoglobin is the main protein within red blood cells and its made of four globin subunits each containing a heme group capable of binding one molecule of O2. This Osmosis High-Yield Note provides an overview of Blood Components and Function essentials.
A A- B B- AB AB- O O-. This figure shows the effects of osmosis on red blood cells. When placing a red blood cell in any hypertonic solution there will be a movement of free water out of the cell and into the solution.
Other major blood components include plasma white blood cells and platelets. Blood is pumped next to a membrane that has dialysis fluid on the other side. Leaf cells of land plants unless it is raining or the humidity.
If placed in a hypotonic solution water molecules will enter the cell causing it to swell and burst. Red blood cells lose water and shrink in a concentrated solution. Osmosis Tonicity and Hydrostatic Pressure.
B C A. Click to see full answer Likewise how can osmosis cause Crenation of red blood cells. The primary function of red blood cells is to transport oxygen to body cells and deliver carbon dioxide to the lungs.
When both classification systems are combined there are eight possible blood types. TaylorIn order to regulate osmosis a cell uses a fluid. Blood Components Function Figure 437 Blood types are reported as ABO group and Rh or -.
Osmosis has a significant role to play in plants animals and also in humans. An early application of the basic principles of osmosis came from the pioneering work on hemolysis of red blood cells by William Hewson in the 1770s see Chapter 2. Draw the solution under the cover slip by using the paper towel.
Unless an animal cell such as the red blood cell in the top panel has an adaptation that allows it to alter the osmotic uptake of water it will lose too much water and shrivel up in a hypertonic environment. Describe what you think happened in the body to result in the three conditions. It is the salt most responsible for extracellular fluid and many multicellular organisms.
In animals the concentration of body. They swell and burst in a solution that is too dilute. The oxygen-hemoglobin dissociation curve shows how the hemoglobin saturation with oxygen SO2 is related to the partial pressure of oxygen in the blood PO2.
In general net movement of water into or out of cells is negligible. These are cells on the underside of leaves that open and close to. Add a drop or two of 3 sodium chloride to the edge of the cover slip.
The absorption of water from the soil is due to osmosis. When red blood cells are placed in a hypertonic solution the higher effective osmotic pressure of the bathing solution compared with the intracellular fluid results in water moving down its osmotic gradient and a net movement of water out of the cell via osmosis. In plants guard cells are also affected by osmosis.

Osmosis In Red Blood Cells Photograph By Science Photo Library
8 4 Osmosis And Diffusion Chemistry Libretexts
What Will Happen When Red Blood Cells Are Placed In Class 12 Biology Cbse
Comments
Post a Comment